Since our formation in 2001, Action Duchenne has invested more than £4m in research, working with more than 15 organisations to fund research across 4 continents. Below are some of the projects that we have supported in the past.
Splicing efficiency project
Enhancement of splicing efficiency between trans‐spliced DMD AAV vectors for the production of full‐length fully functional dystrophin protein.
This is follow-on work from Dr Keith Foster who demonstrated that the triple-transplicing method of gene delivery does work in vitro and Professor Dickson’s will investigate this work further.
The aim of this proposal is therefore to use alternative strategies to improve the splicing efficiency between trans-splicing AAV vectors for the delivery of the DMD gene. The strategies, being equally applicable to both dual and triple trans splicing vectors for mini and full- ength DMD gene delivery, and also to the planned next generation of trans‐splicing vectors.
Genome editing surgery
Development of genome editing surgery with applicability to all living with Duchenne – Royal Holloway University
Dr Linda Popplewell summarised their research project as follows:
“The overarching aim of this project is to develop a gene therapy that requires a single administration with applicability to all Duchenne muscular dystrophy (DMD) patients.
The gene therapy we wish to develop is based on using a special pair of molecular scissors to cut DNA that will allow the insertion of a working copy of the dystrophin gene, delivered using a safe carrier, in a privileged and safe location.
Since we are delivering the dystrophin gene in a safe location, this gene therapy will be applicable to all DMD patients and will allow expression of fully functional dystrophin protein at levels to be of therapeutic benefit. Most importantly, the gene therapy to be developed is permanent in nature, removing the need for repeated long-term administration associated with some other therapies. The benefits of this as a therapy for DMD in terms of cost, immunological and toxicological risk are obvious.
The potential therapeutic implications of the work proposed within the six months of funding include:
• Establishment that knock-in of a working copy into a safe location is possible
• Establishment that this would provide permanent expression of functional dystrophin protein following a single treatment
• Establishment of a means to provide a gene therapy treatment for DMD that would have applicability to all patients”
Action Duchenne’s Director of Research added:
“We are delighted to see the progress made in the area of gene therapy and continue to support all its different approaches. We set this as an early priority in our research strategy, which is constantly under review.”
AAV-U7
Exon skipping converts an out-of-frame mutation into an in-frame mutation leading to an internally deleted but partially functional dystrophin.
This therapeutic approach has demonstrated considerable success using antisense oligonucleotides (AONs), particularly in recent clinical studies. AONs have the enormous advantage of not producing an immune response, but have the disadvantage of having to be regularly injected to maintain therapeutic benefit.
The French group based at the Institute of Myology, one of the leading international centres,have shown that the antisense sequences could be disguised in a small nuclear RNA such as U7snRNA or U1snRNA.
These therapeutic molecules are packaged in adeno-associated viral vectors (AAV), which ensure a permanent production therapeutic antisense in mouse and dog models. A one-shot treatment of AAV-U7 in animal models has been shown to restore some dystrophin expression, which is associated with an improvement in muscle force.
Because most young people living with Duchenne are treated with corticosteroids which might have an effect on therapeutic AAV treatment, the group propose to evaluate benefits of a combined treatment of therapeutic AAVs and corticosteroids in mdx mouse model of DMD (i) to investigate whether increase of muscle strength and better muscle environment state by corticosteroid treatment would slow down the loss of AAV vectors, and (ii) to characterize the corticosteroid mechanism of action and their impact on the muscle machinery.
Stéphanie Lorain, lead investigator from the Institute of Myology said:
“With the first clinical trial planned with this technology to deliver exon skipping planned later this year, this project will give us a better understanding of the potential treatment effect with best standards of care. We are pleased that Action Duchenne are supporting this important project.”
SKIP-NMD (exon 53 programme)
In late 2012, we awarded a small grant to be involved in the SKIP-NMD programme. SKIP-NMD is an EU FP7 funded collaborative grant involving 10 partners from Europe and the USA, whose aim is to restore dystrophin production in a subset of DMD boys. This will be achieved by developing a drug which ‘skips’ exon 53 the mutations causing DMD, so as to restore dystrophin protein expression.
This exon skipping compound is based on Sarepta Therapeutic’s PMO AO chemistry and will potentially treat boys with deletions spanning exons 52, 45-52, 47-52, 48-52, 49-52 and 50-52 (about 6% of the population). The drug will first undergo pre-clinical tests, followed by a phase I/IIa clinical trial. The project will also develop new outcome measures and biomarkers to ascertain the drug’s effectiveness.
PepGen
In memory of the late Hilary Hoskin and in thanks to her trust dedicated to young people like her Grandson, Saul Catlin. The exon skipping project is led by Professor Matthew Wood’s team at the University of Oxford and specifically on Peptide platform technology for enhanced oligonucleotide delivery (PepGen).
The PepGen project aims to develop the next generation of short peptides that attach to antisense oligonucleotides that may improve their delivery to muscle. This will drive forward the development of second generation exon skipping therapies for Duchenne muscular dystrophy. The award will fund the screening of new variants of the delivery in order to progress these potential new compounds into clinical trial as soon as possible.
Professor Matthew Wood says, “We’re delighted to receive this award from Action Duchenne. The award will help us to accelerate the development of peptide technology that improves the delivery of antisense oligonucleotides for exon skipping and make a real impact on the treatment of DMD“.
Janet Hoskin, Founder of Action Duchenne says “We are so pleased that our Mum’s legacy, The Hilary Hoskin Trust, will be funding research that will improve the delivery of exon-skipping drugs to treat Duchenne muscular dystrophy. These are exciting times in the search for genetic treatments; particularly in the light of the FDA’s decision on Dys51 (eteplirsen) and we hope to see more and more options available to those living with Duchenne. Our Mum would be incredibly proud of the way her grandson gets on with life, and we hope that treatments from new biotech start up companies, such as PepGen, will enable him and many others to live longer lives and achieve their aspirations.”
Early research we funded 2001 – 2009
2009 £800,000 AVI Biopharma – The project with the US biopharma has three progammes: Engaging with European and US regulatory authorities to develop exon skipping as a platform medicine,Research and development of other oligos to skip exons 53, 44, 45 and further Development of
European Clinical Trial sites
2009 £35K Appointment of Dr Karen Rafferty, Treat Duchenne Muscular Dystrophy Coordinator. To support the dissemination of internationally agreed Standards of Care for Duchenne to families and clinicians in the UK.
2009 £166K Dr Matthew Wood and Dr Mike Gait – Advances in exon skipping for Duchenne muscular dystrophy: heart correction and multi-exon skipping in partnership with Duchenne lreland
2008 £80K Professor Steve Wilton University of Western Australia – Antisense Oligonucleotide Design in partnership with the James and Matthew Foundation lreland
2008 £20K Dr Mike Gait University of Cambridge, MRC Molecular Biology Laboratory – Development of peptide PNA conjugates for exon skipping
2008 £155K Dr Matthew Wood University of Oxford PNA exon skipping project
2007 £30K Profs. Volker Straub and Kate Bushby, Institute of Human Genetics, Newcastle University, Newcastle upon Tyne, UK – Effect of pharmacologically increased endothelial permeability on the uptake of antisense oligonucleotides in cardiac myocytes in mdx mice
2007 £175K Established the ZF Partnership with leading drug discovery company Summit that has helped to support the further development of a utrophin upregulation drug by Biomarin in the USA. In partnership with Charley’s Fund and Gavriel Meir Trust.
2006 £60K Professor Steve Wilton University of Western Australia – Using explants to develop Exon skipping
2006 £150K Professor Kay Davies University of Oxford Using AAV/U7 to develop exon skipping for Duchenne in partnership with ICE (Monaco and France Duchenne Parent projects)
2005 £ 100K Dr Jenny Morgan and Professor Francesco Muntoni lmperial College and UCL London – Stem cell therapy using a lentivirus to modify the faulty gene
2003 Establishing the MDEX consortium with leading UK scientists and Clinicians in partnership with the Muscular Dystrophy Campaign and Duchenne Family Support Group . Funding of 2.2m secured for the MDEX consortium from the Department of Health and the Medical Research Council
Pilot Trials Now – Sildenafil and IGF-I
Since 2011, Action Duchenne has invested in two programs of the Pilot Trials Now initiative, which ended at the end of 2013.
The first trial testing sildenafil (Revatio or Viagra), originally developed by Pfizer as a heart medication, has been shown to delay and even prevent heart failure in the DMD animal model. The prinicipal Investigator Kathryn Wagner, Kennedy Krieger Institute of Johns Hopkins University led the 12-month, double-blind, placebo-controlled REVERSE DBMD pilot trial to test if Phosphodiesterase 5 inhibition with sildenafil improved cardiac function in adults and adolescents living with Duchenne.
In August 2012, the Food and Drug Administration authority notified healthcare professionals that “use of Revatio, particularly chronic use, in children is an off-label indication, not approved by FDA and is not recommended.” The trial consequently excluded those enrolled under the age of 18.
The full data analysis of the secondary endpoints is still ongoing and is awaiting publication. This was a small study due to Data Safety Monitoring Board recommended closure; therefore this is a cautious interpretation. Overall, Sildenafil was well tolerated in DMD men, but is unlikely to provide benefit to cardiac or skeletal muscle in adult DMD. Sildenafil at 20 mg three times a day for 6 to 12 months may have adverse effects on cardiac function. The study authors concluded that the monitoring of cardiac function in future clinical trials modulating this pathway will be necessary.
The second trial from the Pilot Trials Now scheme was recombinant human insulin-like growth factor-I (IGF-I) therapy in Duchenne. IGF-I was seen as offering potential as a therapeutic agent by improving or preserve muscle function, and countering the effects of glucocorticoid steroid use in some boys, of growth failure and insulin resistance.
In this first study of IGF-I therapy in DMD boys, 6 months of once-daily IGF-I significantly increased height velocity and compared to controls, but there was no difference in motor functional outcomes.
These results were presented at the World Muscle Congress in October 2013 and are currently awaiting acceptance in a leading peer-reviewed scientific journal the European Society of Paediatric Endocrinology.
Halofuginone
Action Duchenne alongside other foundations supported the pre-clinical development of a novel compound called halofuginone, developed by HALO therapeutics. Halo’s HT-100, an investigational anti-fibrotic for Duchenne, is temporarily on hold in the US following a formal action from the FDA requiring Halo to stop dosing and to not enrol new boys in the phase IIa program. The study was placed on hold at the end of December and since their decision the FDA have met with the study team and they expressed their commitment to working with the lead investigators to allow the program to continue as soon as possible.
The target of the investigational compound, fibrosis, with progressive replacement of muscle tissue, is a prominent feature in some muscular dystrophies, preventing complete regeneration and hampering muscle functions. Halofuginone, an inhibitor of Smad3 phosphorylation downstream of TGF signaling, inhibits the activation of fibroblasts and their ability to synthesize the extracellular matrix. In animal models of duchenne and other muscular dystrophies with prominent muscle fibrosis, halofuginone treatment has resulted in both prevention of collagen production in young animals and resolution of established fibrosis in older ones: the reduction in muscle collagen content was associated with improved muscle histopathology and major improvements in muscle function.
Exon skipping with PNAs
Action Duchenne also supported an exon-skipping project by Professor Haifang Yin in China with a three year project, which is shortly coming to an end entitled: “Developing Peptide Nucleic Acid Antisense Oligonucleotides for Duchenne Muscular Dystrophy.”
Since 2006, Haifang Yin and Wood have started to investigate alternative antisense oligonucleotide (AO) chemistries, e.g. peptide nucleic acid (PNA). PNAs are DNA/RNA analogues formed by replacing the sugar phosphate backbone of the native nucleic acid with a synthetic glycine peptide backbone, which is stable and highly resistant to proteases and nucleases with high nucleic acid binding affinity and sequence specificity. The promising data from local and systemic PNA studies indicated that more systematic studies are warranted to fully characterize and explore the clinical potential of PNA AOs for Duchenne. After lots of trials and optimization, Haifang and the team proved the feasibility of synthesizing neutral and clinically applicable PNA AOs and establish the standard protocol, paving the way for developing PNA AOs to clinic. However, the scale-up needs to be further improved for a longer-term.
While the team have been trying the best to establish the therapeutic PNA synthesis platform with a longer horizon to clinic, they have also been exploring other clinically applicable delivery approaches. The team are currently optimising novel formulations that can also facilitate the uptake of AOs in muscle.
Development of triple-transplicing technology using AAV Vectors
In 2011, Action Duchenne invested in the first programme of its kind, using adeno-associated virus via triple-transplicing technology, a form of gene therapy, to deliver the full dystrophin gene. Dr Keith Foster, Reading University, led the project entitled: “Development and evaluation of AAV vectors to restore full length dystrophin to skeletal and cardiac muscle.”
Gene therapy for Duchenne aims to compensate for the lack of dystrophin by transferring the functional dystrophin gene into muscle. Since the DMD gene is the largest gene in the body, delivery of the whole gene is difficult. Shortened versions of the gene have been developed, but full functionality of the protein expressed is compromised as a result of important parts of the protein being missing.
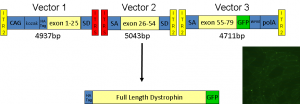
Figure 6 Schematic diagram of triple-hybrid trans-splicing AAV vector system (TTS-AAVs) for expression of full-length dystrophin. The full length codon optimised dystrophin cDNA was cloned into 3 individual AAV vectors such that the reading frame is only re-constituted if there is correct trans-splicing between vector 1, 2 and 3 respectively. (Courtesy of Dr. Keith Foster , Uni. of Reading)
This approach aims to address this problem by dividing the DMD gene into three segments and packaging them into a series of novel gene carriers (known as trans‐spliced adeno-associated virus vectors). The vectors are based on harmless viruses that can carry DNA for delivery to targeted cells within the body. These vectors have been designed so that when used in combination, the different DMD gene segments join together and full length dystrophin protein is expressed (see Figure 6 for more details).
Dr Keith Foster demonstrated proof-of-concept that this methodology can work (data-led evidence that three AAV vectors can be used in concert to deliver full length dystrophin). He started to produce a second-generation viral vector to increase the efficiency of this approach and improve the levels of dystophin expression. He also plans to publish his work in the near future based on the findings of this project that repeat AAV administration results in incremental levels of gene expression.
Exosomes
In November 2013, Action Duchenne, provided funding for one year of an innovative cutting-edge proposal entitled: “Exosomes: a novel therapeutic approach for the treatment of dystrophinopathies.” This project will be led by Dr Mattia Calissano, Newcastle University and expert collaborators Professor George Dickson, Royal Holloway and Francesco Muntoni.
Exosomes are natural lipid particles secreted by a vast variety of cells. By using these particles experimentally loaded with wild type dystrophin protein; the investigators aim to target and restore correct amounts of the dystrophin protein. This approach could offer a new and highly physiological way to restore dystrophin expression and thus functionality in the both the skeletal and cardiac muscles.
Exon skipping in combination with BGP-15
Action Duchenne supported a project with Professor Matthew Wood, Oxford University. This one year contract, which started in September 2013, looked at a small molecule drug (BGP-15) which activated the heat shock response (a hsp72 agonist) and, therefore, has the ability to protect cells (e.g. muscle cells) from stress (such as that resulting from muscle degeneration). This therapy has showed benefit in the mdx mouse and dystrophin and utrophin deficient DKO mouse.
BGP-15 was being investigated in diabetes and a range of other disorders and had been shown to be well-tolerated. Of course such an approach cannot repair or replace dystrophin, but by helping to protect degenerating muscle cells it may help to create a much more favourable environment for other therapies, including exon skipping to work. This pump priming project therefore aims to carry out a preliminary study to determine whether or not BGP-15 has any synergistic benefit when co-administered with current state-of-the-art antisense oligonucleotides for exon skipping, peptide-conjugated PMOs.

