Professor Dickinson’s team have also been involved in developing exon skipping drugs, which selectively target various exons. Exon skipping is a novel therapeutic approach to correct mutations in DMD patients and restore dystrophin expression.
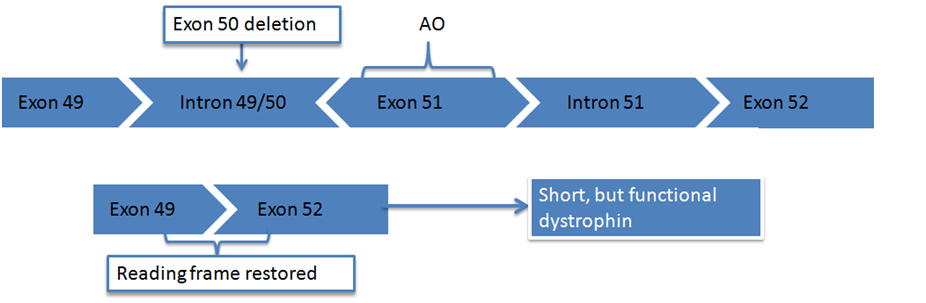
In order to understand the concept of exon skipping, it is first necessary to understand how genes work and how mutations in the dystrophin gene cause Duchenne. A gene is a section of DNA that contains the instructions for the production of one specific protein. Genes are divided into exons and introns. Exons are the sections of the DNA that code for a protein and they are interspersed throughout the DNA alongside introns. When a protein such as dystrophin is being produced, the introns are cut out leaving just the exons. These exons are joined together like pieces of a puzzle. Once the pieces are all put together in the correct order, the body is able to produce the protein.
To produce the dystrophin protein, 79 exons are needed. However in Duchenne some exons are deleted (out-of-frame mutations), and the puzzle doesn’t fit together. Once the cell realises that one exon is missing, the rest of the exons are not read and the production of dystrophin is terminated. A functional protein cannot be produced since the domain connecting it to the connective tissue is lost, and it cannot serve a linker function.
Exon skipping involves persuading the muscle tissue to ignore and ‘skip over’ the faulty puzzle piece in the gene allowing the jigsaw to come together properly, and thus produce the functional dystrophin protein by allowing the rest of the instructions to be properly read.
SKIP-NMD is an international project being funded by Sarepta Therapeutics to utilise exon skipping techniques as a therapeutic option for DMD. The drug being developed, SRP-4053, is an antisense-oligonucleotide(AON) which targets exon 53 of the dystrophin gene, and is currently in phase III of clinical development. This will be beneficial for patients whose condition is a result of deletions of exons 52, 45-52, 47-52, 48-52, 49-52 or 50-52.
Eteplirsen is Sarepta’s leading drug candidate, targeting exon 51, whilst other hopefuls remain in development and preclinical trials.
One obstacle presented by exon skipping is the fact that each person will require a different exon to be skipped. This means that exon skipping is not a ‘one size fits all’ solution. However, data suggests that over 80% of Duchenne patients have genotypes responsive to exon skipping, with potentially 14% responsive to exon 51, 10% responsive to exon 53 skipping, and 9% to skipping exon 45, thus this technique offers a promising approach to novel therapies.
Researchers working on exon skipping face the challenge of standardizing the assessment of dystrophin levels. The aim of these projects is to detect biomarkers in the serum of patients with Duchenne, due to the greater ease of taking blood as opposed to performing muscle biopsies on patients every 6 months.
2 of the major drawbacks that exist with exon skipping are the same delivery issues as those faced with gene therapy, and also the short-lived effects of the treatment which would only last for a few weeks.
AON targets dystrophin RNA. Only muscle cells make dystrophin; in Duchenne these muscle cells are replaced by fibrotic and fat tissue which do not make dystrophin so you need muscle cells in order for this therapy to be effective. Exon skipping will not replace muscle which is already lost, instead it will reduce the rate at which existing muscle is lost. It is important to start treatment early but there are possibilities that it could be combined with drugs to enhance muscle mass for example myostatin inhibitors or steroids.

