Italfarmaco Group announces positive topline data from Phase 3 trial showing beneficial effect of Givinostat in patients with Duchenne muscular dystrophy
This article summarises the results shared by Italfarmaco at the Annual PPMD conference on 25th June 2022. The company presented positive topline data from their EPIDYS, Phase 3 clinical trial evaluating Givinostat in boys with Duchenne muscular dystrophy. Link here
Background/Givinostat target
In normal muscles, a repair mechanism is activated in response to any damage to the muscle fibres. However, in Duchenne muscular dystrophy the lack of dystrophin leads to an increase in the frequency of damage to muscle fibres and results in failure of the repair system. Overtime, due to additional damage and in absence of the repair mechanism, muscle fibres are replaced with scar tissue and fat cells.
Givinostat aims to restore the repair mechanism. The production of some muscle regeneration factors is controlled by how tightly the DNA is packed in our chromosomes. Our DNA is coiled around protein complexes called histones (Figure 1).
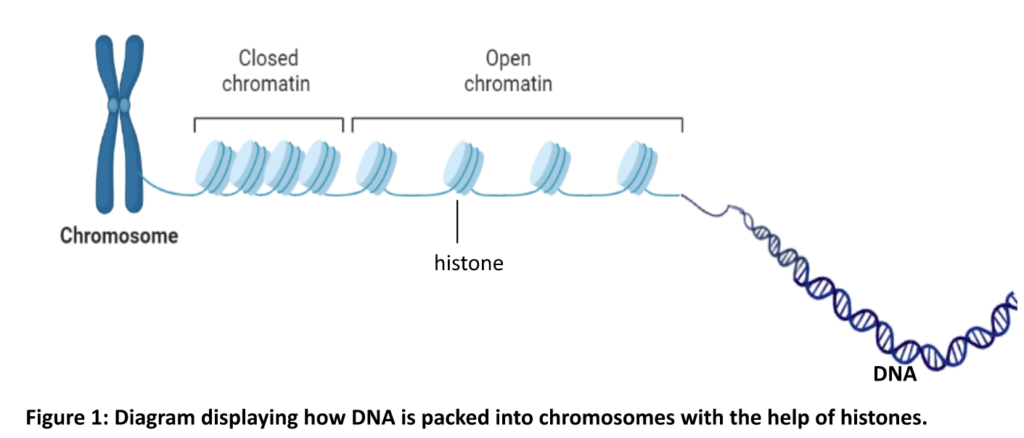
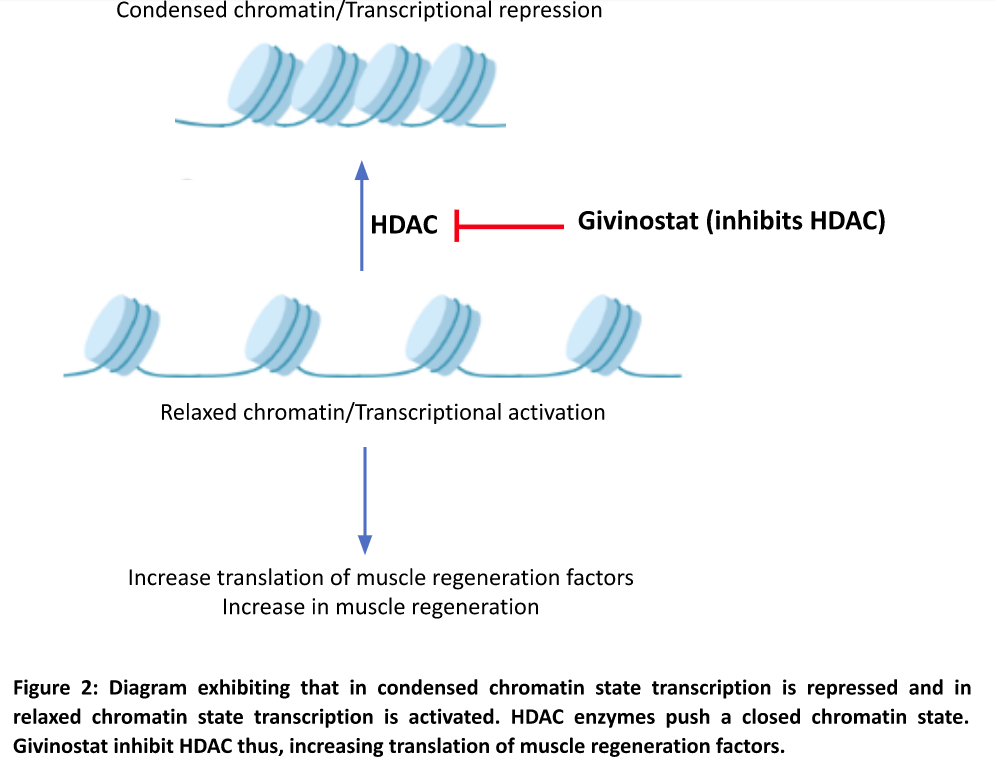
In the condensed/closed chromatin state, transcriptional repression occurs (DNA is not processed further to make downstream proteins). In its relaxed/open state, the DNA coiled around the histones is in a transcriptionally activated state (DNA can be processed to make repair proteins and muscle regeneration factors). Histone deacetylase (HDAC) enzymes push the relaxed chromatin to a ‘closed’ status hence downstream muscle regeneration processes cannot be activated and inflammation is initiated. Givinostat is a HDAC inhibitor and promotes a relaxed chromatin state which leads to increase in translation (process by which protein is made using RNA) of muscle regeneration factors and muscle regeneration (Figure 2).
About Givinostat
Previously, in preclinical mouse models Givinostat showed increase in muscle fibre diameter, decrease in fibrosis and inflammation. A phase 2 study (NCT01761292) assessed the safety and tolerability of Givinostat in children. The phase 2 study showed increase in muscle fibre regeneration, reduction in tissue damage and inflammation with Givinostat given to participants already using steroid therapy. Slower disease progression was also exhibited with Givinostat when compared to historical data.
Givinostat for the treatment of Duchenne muscular dystrophy was granted a Rare Paediatric Disease designation by the U.S. Food and Drug Administration (FDA) in October 2020. Previously, an Orphan Drug Designation from the FDA and EMA, and designation of Fast Track by the FDA was also received by the company.
EPIDYS, Phase 3 clinical trial
EPIDYS clinical trial assessed the efficacy and safety of Givinostat in boys with Duchenne muscular dystrophy (NCT02851797). The trial is double-blinded, randomised and placebo controlled which enrolled 179 ambulant male participants who were on steroids for at least 6 months (mean age was 9 years). The participants were randomised 2 to 1 and treated with oral suspension of Givinostat for or placebo for 18 months.
The overview of clinical results is summarised in the table below:
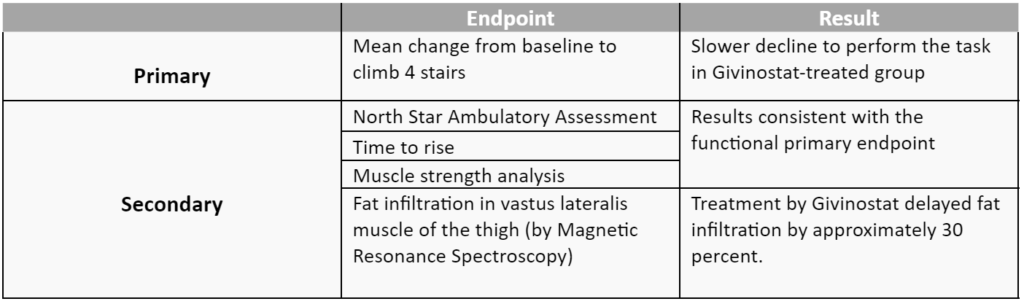
In 95% of the participants the Adverse Events (AE) were mild to moderate in severity, 2.5% (3 boys) withdrew from the clinical trial due to an AE. The AE seen in at least one of the ten participants were abdominal pain, diarrhoea, platelet decrease and triglyceride increase. The AEs were managed with dose alterations and appropriate monitoring.
Conclusion
It is great to see consistent performance of Givinostat over the years and the results are much welcomed in an area where more therapies are needed to preserve muscle fibres and prevent damage. Although Givinostat is not targeting the underlying cause of Duchenne muscular dystrophy or dystrophin but its beneficial therapeutic impact on downstream targets and events due to lack of dystrophin are clear. Italfarmaco is planning to go ahead with potential marketing application submission discussions with the regulatory authorities for the use of Givinostat in Duchenne muscular dystrophy.


 Indoor Skydiving Experience Giveaway!
Indoor Skydiving Experience Giveaway!
